When searching for extraterrestrial life, why do we assume such life would be carbon-based?
Is it possible that life elsewhere might not be carbon-based? Certainly. But it is more likely that it is carbon-based. Why? Chemistry.
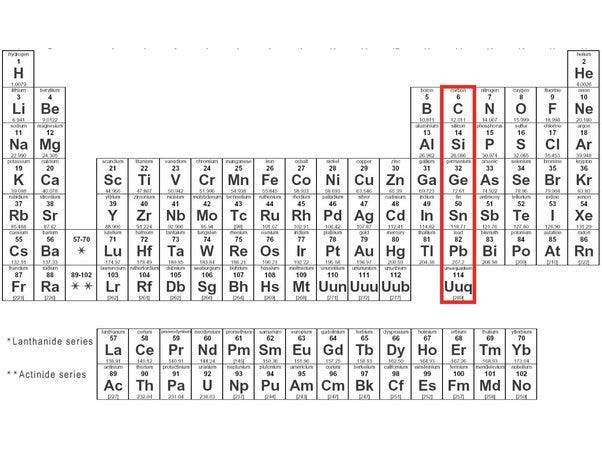
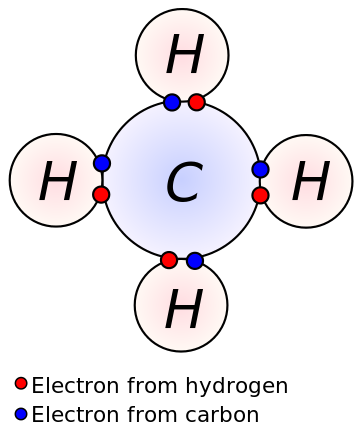
I've highlighted a column in the periodic table, with a red border. Carbon is at the top of this column. Carbon is the lightest, most abundant, element with four valence electrons in a shell capable of eight. That means a carbon atom can form four covalent bonds while nitrogen (to its right) can form three and oxygen (to nitrogen's right) can form two. Here is a diagram of methane, an example of carbon using all four bonds.
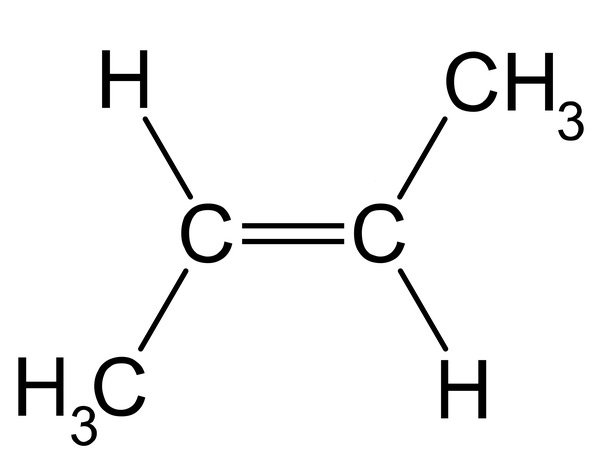
Carbon can also form double bonds, allowing strong (but not so strong the molecules can't change), complex, branching molecules, like this butene molecule.
This means carbon is a light and abundant element capable of forming very complex and flexible molecules. Life is complex. Life needs to be flexible to survive.
But what about the other elements in the red box? Well, there is something called the double bond rule that says period 3 and below do not readily form double bonds. If they do, the bonds are weak. So, the lower we go down that column the less likely life can form based on that element. Silicon can form double bonds with itself, forming diselenes which are unstable. Silicon is a possibility for life, but far less likely than carbon. Science fiction authors like to use it, such as in the Star Trek episode, Devil in the Dark, where the crew of the Enterprise met the silicon-based Horta.
So, the primary reason we look for carbon-based life is that it is more likely to exist. But the secondary reason is that we know what it looks like. There might be other life out there that is not carbon-based, but it might be so foreign to us that we wouldn't recognize it as life.